Raising battery life with silicon all the rage
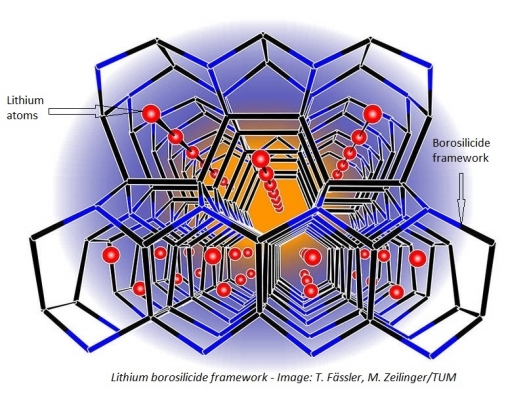
A novel tetrahedral structure consisting of boron and silicon, serving as an electrode material in lithium borosilicide (LiBSi2), forms open channels. This offers, in principle, the possibility to store and release lithium atoms, an important requirement for the anode material used for lithium-ion batteries
Laptops could work longer and electric cars could drive farther if it were possible to further increase the capacity of their lithium-ion batteries.
The electrode material has a decisive influence on a battery's capacity. So far, the negative electrode (or anode), typically consists of graphite, whose layers can store lithium atoms.
Earlier this week we reported on how Stanford University scientists found that lithium-ion battery life could be boosted by using electrodes made of silicon and a conducting polymer hydrogel. (See http://www.siliconsemiconductor.net/article/77527-Silicon-electrodes-boost-lithium-ion-batteries.php )
Now scientists at the Technische Universitaet Muenchen (TUM) have developed a material made of boron and silicon that could smooth the way to systems with higher capacities.
Loading a lithium-ion battery produces lithium atoms that are taken up by the graphite layers of the negative electrode. However, the capacity of graphite is limited to one lithium atom per six carbon atoms. Silicon could take up to ten times more lithium. But unfortunately, it strongly expands during this process - which leads to unsolved problems in battery applications.
Looking for an alternative to pure silicon, scientists at the Technische Universitaet Muenchen have now synthesised a novel framework structure consisting of boron and silicon, which could serve as electrode material. Similar to the carbon atoms in diamond, the boron and silicon atoms in the novel lithium borosilicide (LiBSi2) are interconnected tetrahedrally. But unlike diamond they also form channels.
"Open structures with channels offer in principle the possibility to store and release lithium atoms," says Thomas Fässler, professor at the Institute of Inorganic Chemistry, Technische Universitaet Muenchen. "This is an important requirement for the application as anode material for lithium-ion batteries."
High-pressure synthesis
In the high-pressure laboratory of the Department of Chemistry and Biochemistry at Arizona State University, the scientists brought the starting materials lithium boride and silicon to reaction. At a pressure of 100,000 atmospheres and temperatures around 900oC, the desired lithium borosilicide formed. "Intuition and extended experimental experience is necessary to find out the proper ratio of starting materials as well as the correct parameters," says Thomas Fässler.
Lithium borosilicide is stable to air and moisture and withstands temperatures up to 800 ° C. Since the framework structure of the lithium borosilicide is unique, Fässler and Zeilinger could give a name to their new framework. In honour of their university, they chose the name "tum."
Next, Thomas Fässler and his graduate student Michael Zeilinger want to examine more closely how many lithium atoms the material can take up and whether it expands during charging. Because of its crystal structure the material is also expected to be very hard, which would make it attractive as a diamond substitute as well.
More details of this research has been published in the paper, " LiBSi2: A Tetrahedral Semiconductor Framework from Boron and Silicon Atoms Bearing Lithium Atoms in the Channels," by M. Zeilinger et al in Angewandte Chemie International Edition, 52 (23), p5978-5982. DOI:10.1002/anie.201301540.
The work was funded by the TUM Graduate School, the German Chemical Industry Fund, the German Research Foundation, the Swedish Research Council and the National Science Foundation, USA.

































